- Overview
- Product Description
- Detailed Photos
- Product List
- Company Profile
Basic Info.
Product Description
China Manufacturer Handheld Pulse Oximeter
Model:CMS60D




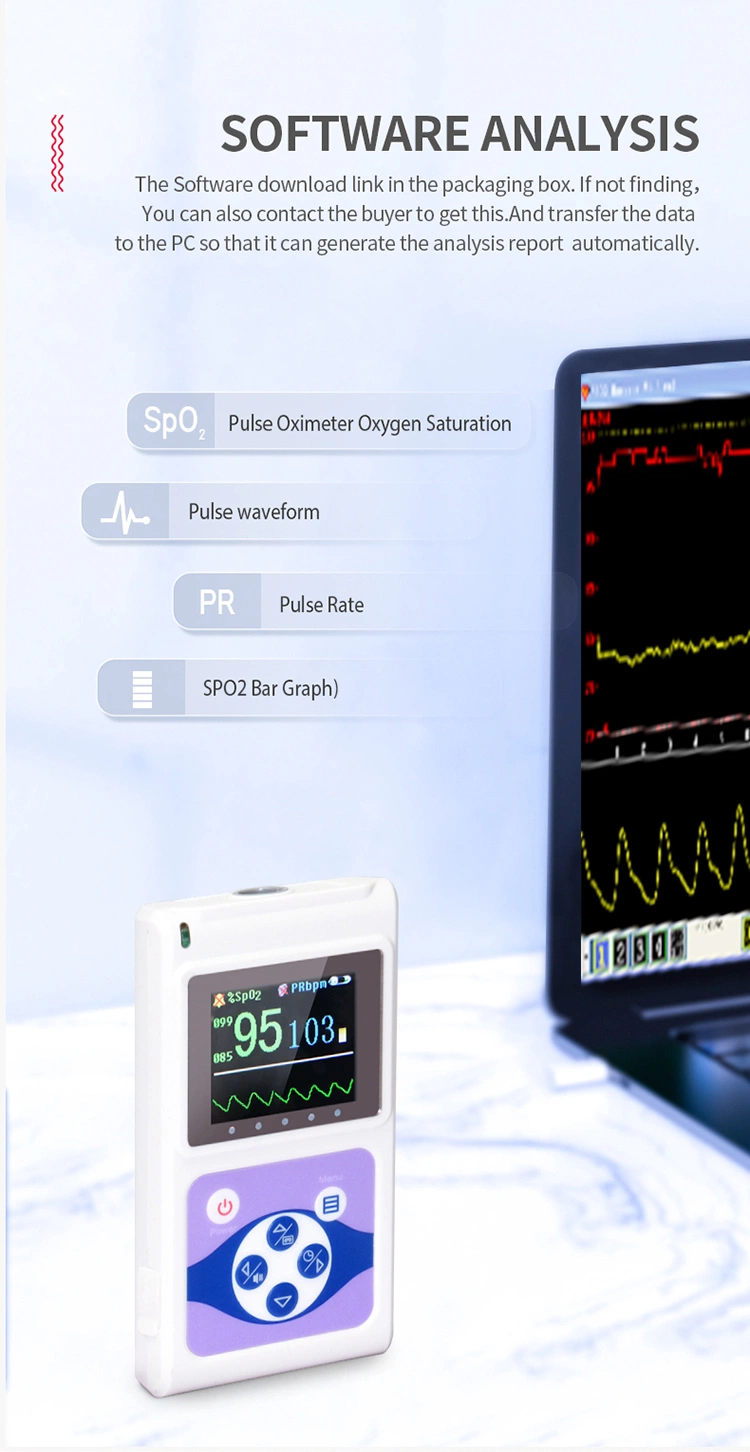



Instructions
Principle of the CMS60D handheld Pulse Oximeter is as follows: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning & Recording Technology,the Pulse Oximeter can be used in measuring the pulse oxygen saturation and pulse rate through finger.The handheld type oximetry is suitable for being used in family, hospital, oxygen bar, community healthcare, physical care in sports (Oximeter can be used before or after doing sports, and CMS60D is not recommended to use the device during the process of having sport) and etc.
Major Features of handheld pulse oximeter
- Small in volume,light in weight and convenient in carrying
- Operation of the product is simple ,low power consumption
- Operation menu for the function setting
- SpO2 value display
- Pulse rate value display, bar graph display
- Pulse waveform display
- Screen brightness can be changed
- Pulse rate sound indication
- With review function
- With clock function
- With measured data overruns limits and low-voltage alarm function
- Battery capacity indication
- Low-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltage ,and with alarm function
- With SpO2 value and pulse rate value of storage, the storage data can be uploaded to computers
- Real-time data can be transmitted to computers
- Connected with an external oximeter probe
Main performance of CMS60D(oximeter)
- Display Mode: 1.8" Color OLED display
- Screen Resolution: 160*128
- SpO2 Measuring Range:0%~100%(the resolution is 1%)
- Accuracy: 70%~100%: ±2%, Below 70% unspecified.
- PR Measuring Range: 30bpm~250bpm, (the resolution is 1bpm)
- Accuracy: ±2bpm or ±2% (select larger)
- Measurement Performance in Weak Filling Condition:SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger).
- Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
- Power Consumption: less than 100mA
- Voltage: DC 2.6V~3.6V
- Power Supply: Dry battery(2AA)
- Battery working hour: The theoretical number is 44 hours.
- Safety Type:Interior Battery, BF Type
Accessories
- Sell in standard(handheld oximeter)
A user manual
Adata line
A disk (PC software)
An adult oximeter probe - Sell in addition
Neonatal probe/Pediatric probe
Physical Identity
Dimension:110(L) × 60(W) × 23(H) mm
Weight:About 120g (with Alkaline battery(2AA))
Certification
CMS60D:Pulse Oximeter -CE , FDA
More information About China Manufacturer of pulse oximeter,fetal doppler supplier/manufactuer,ultrasound scanner supplier/manufactuer,ECG/EEG/EMG manufacturer-CONTEC MEDICAL SYSTEMS CO., LTD



-TELEMEDICINE products
-Patient Monitoring
-ECG machine(Electrocardiograph)/Holter ECG/Stress test ECG,
-EEG,
-Ultrasound Imaging,Pocket fetal doppler,fetal monitor,
-Blood pressure Monitor(ABPM),
-spirometer
-infusion/syringe Pump
-Digital Stethscope
-In-Vitro Diagnostics
-simulator
-Veterinary products
View more products,click here...
| Business Type | Manufacturer, Trading Company | Country / Region | Hebei, China |
| Main Products | Pulse Oximeter, Pocket Fetal Doppler, Patient Monitor, ECG, Ultrasound Imaging | Ownership | Public Company |
| Total Employees | Above 1000 People | Total Annual Revenue | 2009943846 USD |
| Year Established | 1996 | Certifications(6) | ISO13485, FSC, ISO14001, Other, ISO9001, ISO13485 |
| Product Certifications(2) | CE, RoHS | Patents | Y |
| Trademarks | CONTEC | Main Markets | Domestic Market30.00% North America18.00% Western Europe15.00% |
Contec Medical Systems Co., Ltd. (hereinafter r is a high-tech company which focuses on research, manufacture and eferred to as CONTEC)distribution of medical devices since 1996, CONTEC locates in beautiful seaside city, Qinhuangdao, and is one of the largest bases for R&D and manufacturer of medical devices in China.
In August 2020, CONTEC officially became a listed company in Shenzhen Stock Exchange (Stock Code:300869).
CONTEC has more than 1,000 employees, covering an area of 67,450 square meters. we have a complete R&D, production and sales system, also we have got ISO9001, ISO13485 quality management system certification, ISO14001 environmental management system certification, BSCI Business Social Compliance Initiative and many other international standards Certification.
The company has strong technical force and has obtained a number of core technology patents with independent intellectual property rights. Since its foundation, CONTEC is dedicated to develop and expanding our line of medical devices, till now we have more than 20 categories of products containing Pulse Oximeter, Sphygmomanometer, ECG, EEG, Ultrasound Equipment, Patient Monitor and Image Equipment, etc.,, and we own more than 100 national patent, 56 software copyright, our products have got CE, FDA and Canada/Japan/Brazil approval. Now, CONTEC products were distributed to over 130 countries and regions.
People-oriented, innovative and win-win is CONTEC's development philosophy, CONTEC will take its advantage on technology and talent, furthermore promote its market internationalization, modern management, product diversification, and endeavors to build CONTEC a world-class enterprise and achieve sustainable & healthy development.